Lab convection in gases and liquids will both expand
This easily leads to other applications such as transfer lines for liquefied gases that are surrounded by an annular tube with a vacuum between the tubes to prevent heat from reaching the liquid and causing vaporization. As with mechanical effects from pressure differentials, thermal conductivity can be used as a pressure gauging technique where a wire is heated by the application of constant power and the heat loss of the wire is indicated by its temperature which is either measured directly thermocouple gauge or by its resistance pirani gauge.
The same effects as found with thermal conductivity can also be applied to sound or electrical insulation with some specific complex differences. Virtually any material will vaporize if you get it hot enough, but vacuum processes tend to be mostly concerned with the vaporization of fluids.
A fluid that has any volatility at all at room temperature will vaporize sooner or later at atmospheric pressure. In terms of a practical process, sooner will usually be better than later, so means to increase the vaporization rate can be important.
Since vaporization is really molecules of the liquid leaving the surface and not returning as liquid, increasing the rate of vaporization will mean increasing the number of molecules leaving in a given time. At atmospheric pressure, the rate of loss will be relatively slow because of the high number of molecules directly above the surface. This means that a vaporizing molecule will probably immediately impact a gas molecule, lose its energy, and return to the liquid state.
If, however, the liquid is within a chamber that has been evacuated to some extent, fewer molecules will be above the surface. This means that a vaporizing molecule will have a lesser chance of impacting a gas molecule because there are fewer molecules to hit, bigger spaces between molecules, and fewer molecules impacting the liquid surface.
A practical example would be the difference in the boiling point of water between valley and mountaintop. When a liquid boils, it has reached a critical point where the heat being added to the liquid is instantly translated into vaporization so the temperature of the liquid will not change.
As altitude is increased, the pressure is reduced so there are fewer molecules the inhibit vaporization and less energy is required for the vaporizing molecules to overcome the collisional losses from the ambient gas molecules. Figure 4 shows the difference in the boiling point of water at various altitudes. A practical vacuum process would be vacuum distillation where it is necessary to separate two liquids with different vapor pressures.
Flowing a film of the liquid mixture into an evacuated container at a fixed temperature would force or allow the most volatile liquid to vaporize at a low temperature because fewer molecules would be available to inhibit vaporization than would be present at atmospheric pressure. Hence, fast distillation for a practical process. An example of this kind of process would be the distillation of mechanical pump oil where it is necessary to remove the high vapor pressure volatile components before it can be used in a vacuum pump.
Chemical effects come into play in most cases where the chemical reactivity and properties of the gases will either help or inhibit a process. This often concerns not only the particular gases in question, but also their respective concentrations. Any container, chamber, or plumbing will have been exposed to atmospheric air at some time during its history. Before any of these are used to contain or transfer pure process gases, they need to be evacuated to minimize the detrimental effects of the gases inside before the pure gas is introduced.
If this were not done, it would be much like pouring a purified chemical solution into a dirty beaker. The degree of purity of the gas required would dictate the ultimate vacuum that was necessary since the population of the residual gases would all be considered as contaminants. For example, an oxygen pressure of 10 -3 torr would result in a contamination level of 1 PPM if the container was backfilled to atmospheric pressure with pure gas.
Hence, the total number of molecules in the container would be the main concern in this type of effect. A practical application to illustrate this effect would be the venerable incandescent light bulb which would be evacuated prior to backfilling with inert gas.
Since many materials are subject to chemical reactions with air, it becomes necessary to remove molecules from the vicinity of the surface being processed. The only reasonable way to do this is to place the material in a chamber and use the atmosphere, or lack of it, to protect the material from a chemical reaction.
In this case, the number of molecules of chemically reactive gas impacting the surface would be of prime concern. In some processes, the chamber would be evacuated using the same concerns discussed above in that the chamber would be backfilled with inert gas following evacuation to remove as many reactive gas molecules as the process dictated. In others, the entire process would take place under vacuum to protect the material from chemical reaction.
Metallurgical processes are prime examples where a furnace might be evacuated and then backfilled with inert gas or hydrogen reducing atmosphere or the entire thermal process might be carried out while the chamber was under dynamic pumping conditions. In fact, such processes as vacuum brazing are often a combination in that the furnace, following initial evacuation, is pre-heated while filled with a hydrogen pressure, and just prior to reaching brazing temperature, the chamber is evacuated and then given a temperature spike to the brazing alloy flow temperature.
Thin film processes are prime examples of those processes that require a combination of physical and chemical effects. Consider the simple example of filamentary evaporation of aluminum onto a substrate as shown in Figure 5. A stranded tungsten filament is directly heated by high current until an aluminum staple melts and wets the filament.
If the molecular concentration of chemically active gases is too high, the filament will oxidize and burn out. Additionally, the hot aluminum will oxidize. In this case, the number of impacts of active gas molecules with the surface are the prime concern. Although the heating could be done under an atmosphere of inert gas, this is only a segment of the full set of process parameter requirements.
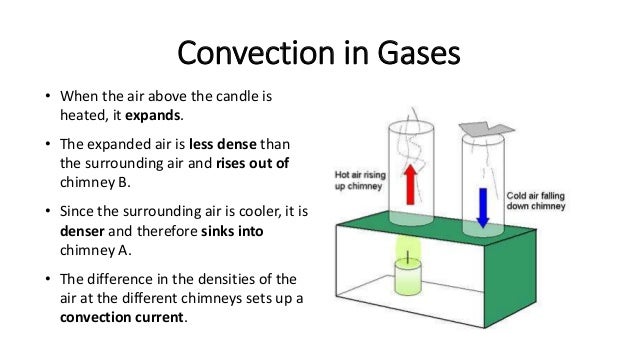
Liquids and gases are fluids. The particles in these fluids can move from place to place. Convection occurs when particles with a lot of heat energy in a liquid or gas move and take the place of particles with less heat energy. Heat energy is transferred from hot places to cooler places by convection.
Your web browser does not have JavaScript switched on at the moment. For information on how to enable JavaScript please go to the Webwise site. Liquids and gases expand when they are heated. This is because the particles in liquids and gases move faster when they are heated than they do when they are cold. As a result, the particles take up more volume.
This is because the gap between particles widens, while the particles themselves stay the same size. The liquid or gas in hot areas is less dense than the liquid or gas in cold areas, so it rises into the cold areas. The denser cold liquid or gas falls into the warm areas.